Journal of Veterinary Diagnostic Investigation
The Journal of Veterinary Diagnostic Investigation is the official journal of the American Association of Veterinary Laboratory Diagnosticians. The mission of the Journal is to educate by informing readers of progress in veterinary laboratory medicine and related fields of endeavor. The key objectives of the JVDI are to promote the science of veterinary laboratory medicine and the betterment of animal and public health.
Three manuscript formats are accepted for review: Review Articles, Full Scientific Reports, and Brief Reports. Review articles are strongly encouraged provided they cover subjects of current and broad interest to veterinary laboratory diagnosticians.
JVDI also publishes position announcements for employment and advertisements for diagnostic products.
JVDI content is open access after a 12-month embargo.
This journal is a member of the Committee on Publication Ethics (COPE)
The American Association of Veterinary Laboratory Diagnosticians (AAVLD) recognizes and fully accepts the need for editorial independence of the Association’s journal, the Journal of Veterinary Diagnostic Investigation (JVDI), and grants the editor-in-chief full authority over the editorial content of the journal, including the type, selection, format, and timing of content for publication. For these purposes, editorial content is understood to include review articles, full scientific reports, brief communications, special reports and consensus statements, editorials, letters to the editor, features, news, and advertising. Opinions and statements expressed in JVDI are those of the contributors and, unless so stated, do not represent the official policy of the AAVLD. AAVLD management does not interfere in the evaluation, selection, or editing of content published in JVDI, either directly or by establishing an environment that has an impact on decisions of the editor-in-chief.
The Journal of Veterinary Diagnostic Investigation (J Vet Diagn Invest) is an international peer-reviewed journal published bimonthly in English by the American Association of Veterinary Laboratory Diagnosticians (AAVLD). JVDI is devoted to all aspects of veterinary laboratory medicine. The major disciplines are anatomic pathology, bacteriology/mycology, clinical pathology, epidemiology, immunology, laboratory information management, molecular biology, parasitology, public health, toxicology, and virology.
Three manuscript formats are accepted for review: Review Articles, Full Scientific Reports, and Brief Communications. Review articles are strongly encouraged provided they cover subjects of current and broad interest to veterinary laboratory diagnosticians. The suitability of Letters to the Editor, Book Reviews, and Commentaries is determined by the Editor-in-Chief, and a pre-submission inquiry to the editor is recommended.
| M. Grant Maxie | Guelph, Ontario, Canada |
| Holly M. Farrell | AAVLD, USA |
| M. Grant Maxie | Guelph, Ontario, Canada |
| Asli Mete | University of California, Davis, USA |
| Francisco A. Uzal | University of California, Davis, USA |
| Pat J. Blackall | University of Queensland, Australia |
| Jérémie Korchia | Michigan State University, USA |
| Steve Bolin | Michigan State University, USA |
| Kathy L. Toohey-Kurth | University of California, Davis, USA |
| Stephen B. Hooser | Purdue University, USA |
| Jianqiang Zhang | Iowa State University, USA |
| Francisco A. Uzal | University of California, Davis, USA |
| Donal O'Toole | University of Wyoming, USA |
| Jeremiah T. Saliki (2004-2014) | |
| John M. Kreeger (1997-2003) | |
| Lenn Harrison (1991-1996) | |
| Robert A. Crandell (1987-1990) |
| Olaf Berke | University of Guelph, Canada |
| Mohamed F. Abdul-Careem | University of Calgary, Canada |
| Shawn Babiuk | Canadian Food Inspection Agency, Canada |
| Udeni B. Balasuriya | Louisiana State University, USA |
| Barbara Banco | Laboratorio Analisi La Vallonea, Italy |
| Timothy V. Baszler | Washington State University, USA |
| Uriel Blas-Machado | University of Georgia, USA |
| Yugendar Reddy Bommineni | Bronson Animal Disease Diagnostic Laboratory, USA |
| Grant N. Burcham | Purdue University, USA |
| Glenn H. Cantor | Bristol-Myers Squibb, USA |
| Douglas Segalla Caragelasco | Pontificia Universidade, Brazil |
| Francisco R. Carvallo | Virginia-Maryland Regional College of Veterinary Medicine, USA |
| Anthony W. Confer | Oklahoma State University, USA |
| Rocio Crespo | North Carolina State University, USA |
| Nicola Decaro | University of Bari, Italy |
| Angela Di Cesare | University of Teramo, Italy |
| Misty A. Edmondson | Alabama Dept of Agriculture and Industries, USA |
| Angela Ellis | Antech, USA |
| Bonto Faburay | Kansas State University, USA |
| Pamela J. Ferro | Texas A&M University, USA |
| Geoffrey T. Fosgate | University of Pretoria, South Africa |
| Timothy S. Frana | Boehringer Ingelheim Vetmedica, USA |
| Luis G. Gimenez-Lirola | Iowa State University, USA |
| Katherine Hughes | University of Cambridge, UK |
| Binod Jacob | Merck Pharmaceuticals, USA |
| Subhashinie Kariyawasam | The University of Florida, USA |
| Melissa A. Kennedy | University of Tennessee, USA |
| Peter D. Kirkland | Elizabeth Macarthur Agriculture Institute, Australia |
| Samantha Lee | University of Illinois, USA |
| Panayiotis Loukopoulos | University of Melbourne, Australia |
| Margaret A. Miller | Purdue University, USA |
| Patti J. Miller | University System of Georgia, USA |
| Akinyi C. Nyaoke | University of California, Davis, USA |
| Jane Oakey | Biosecurity Queensland, Australia |
| Joaquin Ortega Porcel | CEU Cardinal Herrera University, Spain |
| Mitchell V. Palmer | Bacterial Diseases of Livestock Research Unit, USDA, USA |
| Viju V. Pillai | South Dakota State University, USA |
| Brandon L. Plattner | Kansas State University, USA |
| Roman Pogranichniy | Kansas State University, USA |
| Julia Ridpath | Ridpath Consulting, USA |
| Franklin Riet-Correa | National Institute of Agricultural Research, Uruguay |
| Nicholas A. Robinson | Bluebird Bio, USA |
| Susan Sanchez | University of Georgia, USA |
| Susan K. Schommer | University of Missouri, USA |
| Ðurda Slavic | University of Guelph, Canada |
| Leyi Wang | University of Illinois at Urbana-Champaign, USA |
| Moges W. Woldemeskel | University of Georgia, USA |
| Yoshinao Yamazaki | ASAHI Veterinary Clinic, Japan |
| David H. Zeman | South Dakota State University, USA |
Click here to view the Instructions for Authors PDF
Contents
3.2 Adequacy of experimental design, test validation, and statistical analysis.. 7
3.3 Adequacy of title, references, figures, and tables......................................... 8
4.1.5 Immunohistochemistry....................................................................... 10
4.1.5 Sources and manufacturers................................................................. 11
APPENDIX A: PCR guidelines—suggested dossier or publication information. 23
APPENDIX C: Abbreviations that may be used without expansion.................... 27
Manuscript submission checklist
- Please note the page charge of $75 for each printed page published in JVDI.
- Please take the time to read our Best Practices for submitting, reviewing, and publishing in JVDI.
- Microsoft Word file, double-spaced, 12-point Times New Roman font, left-justified, 25 mm (1 in.) margin on all sides, pages numbered at the bottom center (i.e., Page X of Y).
- For styles, do not use Heading 1, etc. Use the Normal style setting.
- Number text lines consecutively throughout the manuscript; begin page 1 with line 1; do not restart numbering on each subsequent page.
- Indent paragraphs at 0.5 in (2.54 cm); do not include spaces between paragraphs.
- Allow 1 space (not 2) after a word or period.
- JVDI number style is one, 2, 3, 4… within the text, but 1, 2, 3, 4… when in a series in the same sentence.
- Include tables in the main document, but do not embed figures.
- SI units of measurement (International System of Units) must be used (may include conventional units in brackets; see section 4.1.3).
- For anatomic terms, use the English equivalents of terms in Nomina Anatomica Veterinaria. Names of infectious agents should follow the current published standards for viruses (ICTV, International Committee on Taxonomy of Viruses), bacteria (NCBI), and fungi (NCBI).
- Cite the brand and manufacturer in parentheses at the appropriate location within the text. Do not include city or state.
- Reference citations in text are listed as superscripts after the punctuation, as shown.1,2-4,8
- Arrange references alphabetically, numbered consecutively. See section 4.2.4, References.
- Submit figures in .tiff or .jpg formats only, preferably in color, if applicable (see section 5). Do not exceed the maximum file size of 5 MB per figure.
- Submit supplemental tables as Microsoft Word files (see section 6). Submit supplemental figures following the figure guidelines (see section 5).
1 Scope and editorial policy
The Journal of Veterinary Diagnostic Investigation is the official journal of the American Association of Veterinary Laboratory Diagnosticians. The mission of the Journal is to educate by informing readers of progress in veterinary laboratory medicine and related fields of endeavor. The key objectives of the JVDI are to promote the science of veterinary laboratory medicine and the betterment of animal and public health. JVDI fully supports diversity, equity, and inclusion in our publishing activities.
JVDI is devoted to all aspects of veterinary laboratory medicine. The major disciplines are anatomic pathology, bacteriology/mycology, clinical pathology, epidemiology, immunology, laboratory information management, molecular biology, parasitology, public health, toxicology, and virology. For more information about JVDI, please visit https://journals.sagepub.com/home/vdi
Three manuscript formats are accepted for review: Reviews, Full Scientific Reports, and Brief Reports (see section 4.2.4.). Review articles are strongly encouraged provided they cover subjects of current and broad interest to veterinary laboratory diagnosticians. The suitability of Letters to the Editor, Book Reviews, and Commentaries is determined by the Editor-in-Chief, and a pre-submission enquiry to the editor is recommended.
1.1 Copyright considerations
JVDI accepts original manuscripts for consideration with the understanding that the same material or a substantial part thereof is not presently being considered for publication or has not been published elsewhere. The Corresponding Author must secure the approval of all authors and institution(s) where the work was carried out. A statement to the Editor confirming that such approval has been received must be included in the submission cover letter. Upon acceptance for publication, authors will receive a link to the Contributor Form to transfer copyright or another suitable arrangement to the publisher. All articles published in JVDI are protected by copyright that covers the translation rights as well as the exclusive rights of AAVLD to reproduce and distribute the articles. JVDI will not publish any manuscript for which the signed Contributor Form has not been submitted.
If your manuscript incorporates any previously copyrighted material that is not in the public domain, you must obtain written reprint permission from the copyright owner and submit a scanned PDF of the permission along with your manuscript files via our online manuscript submission portal (see section 8). No manuscript containing previously copyrighted material will be accepted for review in JVDI without submission of satisfactory proof that copyright permission has been obtained.
1.2 Ethical considerations
1.2.1 Animal welfare
JVDI requires that authors obtain the relevant national/state/institutional approval prior to animal experimentation. In the United States, this means the Institutional Animal Care and Use Committee for approval for any animal experiment.
Authors are encouraged to register their clinical trials at https://www.clinicaltrials.gov/ or other suitable databases identified by the International Committee of Medical Journal Editors.
1.2.2 Plagiarism
JVDI employs the software program iThenticate to detect plagiarism. From the U.S. Office of Research Integrity, plagiarism of text is “copying a portion of text from another source without giving credit to its author and without enclosing the borrowed text in quotation marks” and plagiarism of ideas is
“appropriating someone else’s idea (e.g., an explanation, a theory, a conclusion, a hypothesis, a metaphor) in whole or in part, or with superficial modifications without giving credit to its originator.” Detection of plagiarized material in a manuscript will result in its immediate rejection, regardless of its scientific merit. The author’s institution may be notified.
1.3 Authorship
The JVDI Authorship Form is a downloadable PDF that must be completed and submitted during the submission process. For information on ORCID iDs, see section 7.
Manuscripts should only be submitted for consideration once consent is given by all contributing authors. Submitting authors should carefully check that all those whose work contributed to the paper are acknowledged as contributing authors. The list of authors should include all those who can legitimately claim authorship. This is all those who:
- Made a substantial contribution to the concept or design of the work; or acquisition, analysis, or interpretation of data.
- Drafted the article or revised it critically for important intellectual content.
- Approved the version to be published.
- Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Authors should meet the conditions of all of the points above. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
When a large, multicenter group has conducted the work, the group should identify the individuals who accept direct responsibility for the manuscript. These individuals should fully meet the criteria for authorship.
Acquisition of funding, collection of data, or general supervision of the research group alone does not constitute authorship, although all contributors who do not meet the criteria for authorship should be listed in the Acknowledgments section.
For more information, see the International Committee of Medical Journal Editors on the roles and responsibilities of authors and contributors.
Please note that AI chatbots, for example ChatGPT, should not be listed as authors. For more information see the policy on Use of ChatGPT and generative AI tools.
Corresponding author
The one individual who takes primary responsibility for communication with the journal during the manuscript submission, peer review, and publication process, and typically ensures that all the journal’s administrative requirements, such as providing details of authorship, ethics committee approval, clinical trial registration documentation, and gathering conflict of interest forms and statements, are properly completed, although these duties may be delegated to one or more co-authors.
The corresponding author is the person who signs the Contributor Form on behalf of all of the authors and whose contact details are included on the article. They should be available after publication to respond to critiques of the work and cooperate with any requests from the journal for data or additional information should questions about the paper arise after publication.
Equal credit for authorship.
On the title page, use an asterisk (*) after author names to indicate if authors should receive equal credit—no more than 2 authors per manuscript. Below the corresponding author information, add the following text: *These authors contributed equally to this work.
Changes in authorship.
“Any requests for changes in authorship after initial manuscript submission and before publication should be explained in writing to the editor in a letter signed by all authors, or if sent by email, all authors should be copied (i.e., included as recipients of the email).” (Christiansen S, Flanagin A. What’s new in AMA style? Am Med Writers Assoc Ann Meet; Nov 2019; hereafter AMA Manual of Style, 11 ed.)
Third party submissions
Where an individual who is not listed as an author submits a manuscript on behalf of the author(s), a statement must be included in the Acknowledgements section of the manuscript and in the accompanying cover letter. The statements must:
- Disclose this type of editorial assistance—including the individual’s name, company, and level of input.
- Identify any entities that paid for this assistance.
- Confirm that the listed authors have authorized the submission of their manuscript via third party and approved any statements or declarations (e.g., conflicting interests, funding, etc.).
Where appropriate, Sage reserves the right to deny consideration to manuscripts submitted by a third party rather than by the authors themselves.
1.4 Preprints
As part of the submission process, you will be required to warrant that you are submitting your original work, that you have the rights in the work, that you have obtained and can supply all necessary permissions for the reproduction of any copyright works not owned by you, that you are submitting the work for first publication in the Journal, and that it is not being considered for publication elsewhere and has not already been published elsewhere. Note that JVDI may accept submissions of papers that have been posted on preprint servers; include the DOI for the preprint in the designated field during the submission process. Authors should not post an updated version of their paper on the preprint server while it is being peer-reviewed. If the article is accepted for publication, the author may re-use their work according to Sage’s author archiving policy. If your paper is accepted, you must include a link on your preprint to the final version of your paper.
2 Fees and open-access policy
Authors will not be asked for any payment related to their submitted articles until the article is accepted and in production. Please report any suspicious communications you receive to the Managing Editor at editorial@aavld.org
JVDI is online-only for AAVLD members. Once an article has been accepted and files received into Sage’s SMART program, authors will receive an email inviting them to choose a publication route: open-access or routine publication.
Routine publication. There is a page charge of $75 for each typeset page published in JVDI. For color figures, authors are given the choice of online-only (free) or print & online ($800 for the first page of color figures, then $200 for each additional page). Black & white images do not incur any additional cost. Payment is expected within 30 days.
Open-access publication. Open access publishing is offered via the Sage Choice program. The openaccess fee is $3,750 + page charges, and includes free color figures online. Authors are responsible for paying the cost of publishing additional color pages in print at the rate of $800 for the first page of color figures, then $200 for each additional page, and these will be invoiced separately. Black & white images do not incur any additional cost. Payment is expected within 30 days.
Waiver of page charges. JVDI has a limited fund available from the AAVLD Foundation to help researchers from resource-limited countries overcome financial barriers to publication. Authors may apply for a waiver of page charges at the time of submission. Waivers depend on the location of the authors’ institution. First authors with primary affiliations based in countries defined by the World Bank as “Low-Income Economies” (group A) can apply for a 100% page-charge waiver. First authors with primary affiliations based in countries defined by the World Bank as “Lower-Middle-Income Economies” (group B) can apply for a 50% discount on the usual page charge. If accepted for waiver status, the proffered manuscript will proceed through the usual peer-review process. NOTE: At submission, if you are applying for a waiver, please explain in your cover letter how you qualify.
For information on funding body compliance, and depositing your article in repositories, please visit Sage Publishing Policies on the Sage Journal Author Gateway.
3 Review and acceptance criteria
Based on an assessment by the JVDI editorial office of compliance with JVDI acceptability criteria, manuscripts will be reviewed by 2 or more persons selected by the Editors based on their expert knowledge and/or experience in the subject matter. Authors may provide the editorial staff with a list of suggested reviewers for their work and may also request that specific individuals be excluded as reviewers because of potential conflicts of interest. The peer-review process is single anonymized, that is, editors and reviewers are aware of the authors’ identities, but authors are not informed of the reviewers’ identities. Acceptance of a manuscript for publication is determined by the Editors based on peer review, scientific merit, and value to JVDI readers.
Prior publication of an abstract or poster presented at a conference will generally not impact the manuscript’s eligibility for publication.
Potential reviewers of all manuscripts submitted to JVDI are asked to consider any potential conflicts of interest they may have before agreeing to review a manuscript. We expect that reviewers with a substantial conflict of interest will disqualify themselves from reviewing a manuscript. More information, as well as general Instructions to Reviewers, is available from Sage Journal Reviewer Gateway.
Review and acceptability criteria include, but are not limited to, the following: novelty/usefulness and adequacy of experimental design, tables, and figures.
3.1 Novelty of contents and impact/usefulness to veterinary laboratory diagnosticians
3.1.1 Novelty
JVDI is devoted to the publication of original work. Before submitting a manuscript for review, it is the responsibility of all authors to review the literature to ensure that work similar to their own has not been published previously. Authors, please take note of the following examples:
- The development of detection assays (notably PCR procedures) for pathogens that have existing assays already published in the literature: JVDI will consider these papers if it can be demonstrated that the submitted manuscript constitutes a significant improvement over published methodology. Please note that if one or more previously described PCR procedures for any given pathogen exist, subsequent submissions will be acceptable for publication only if the authors demonstrate equivalency (or preferably superiority) via a side-by-side comparison between the existing assay and the new assay. The manuscript must also assess important test-related criteria such as sensitivity, specificity, accuracy, robustness, rapidity, throughput, and cost (see Appendix A).
- The isolation and/or identification of infectious agents from host species that have previously been described in the literature will be considered if the submitted manuscript adds impactful new information, such as a new detection method, novel virulence or pathogenicity data, or unique antimicrobial susceptibility information. In contrast, the detection of a well-known pathogen in a new animal species is not considered sufficiently novel information to warrant publication.
- Single case reports will be considered for publication only if they demonstrate excellence in the diagnostic investigation, including a detailed discussion on the differential diagnosis. Preferred submissions would be novel, emerging, or unique case reports; case series summaries; classic diseases that have significantly evolved or changed in some fashion; or demonstration of the usefulness of new technologies to the diagnostic process. Case reports combined with a review of the literature on the topic of the case report may increase the chances of having a manuscript accepted for publication.
3.1.2 Usefulness and impact
The target readership of JVDI is veterinary laboratory diagnosticians. The contents of manuscripts published in JVDI must be applied science and relevant to the professional activities of this core group. Examples of manuscripts that do not fit within the scope of JVDI include the following:
- Clinically oriented manuscripts regarding therapy and clinical diagnostic techniques (e.g., ultrasonography, radiology).
- Basic science manuscripts (e.g., mapping genes of infectious agents without a practical diagnostic application).
3.2 Adequacy of experimental design, test validation, and statistical analysis
The experimental design used must be appropriate and adequate. Similarly, the interpretations and conclusions must be valid and supported by appropriate statistical analysis.
- All tests used are expected to have been verified or validated in-house, as appropriate. Variants of standard tests may be referenced to a previous peer-reviewed report. No reference is needed for standard techniques, such as tissue sectioning and H&E staining. If standard tests are modified, the authors must present comparability data or reference published data that show comparability.
- From the AAVLD Requirements for an Accredited Veterinary Medical Diagnostic Laboratory, v2021-01
- Validation: The act of confirming, through objective evidence, that the requirements which define an intended use or application have been met. The process through which a test method is confirmed to be fit for the intended purpose.
- Verification: The process of determining accuracy using a reference standard; e.g., comparing the accuracy of a piece of equipment to a NIST calibrated reference standard; the internal process to determine if a laboratory can perform a validated assay and obtain expected results.
- For further information, see: Arnold JE, et al. ASVCP Guidelines: Principles of Quality Assurance and Standards for Veterinary Clinical Pathology (version 3.0). Vet Clin Pathol 2019;48:542–618.
- If the subject of the article is the validation or modification of a PCR assay, then the following information should be described: PCR primer and probe sequences; analytical sensitivity and specificity performance characteristics; repeatability and reproducibility; and diagnostic sensitivity and specificity (Toohey-Kurth K, et al. Suggested guidelines for validation of real-time PCR assays in veterinary diagnostic laboratories. J Vet Diagn Invest 2020;32:802–814; see Appendix A). Alternatively, the author may follow the most recent revision of the OIE validation algorithm
(Principles and methods of validation of diagnostic assays for infectious diseases. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE, 2019). Novel molecular assays without a PCR step and non-molecular tests should follow the most recent revision of the OIE validation algorithm.
- For clinical pathology test guidelines, see current ASVCP Quality Assurance and Laboratory Standards Guidelines. Authors should state which guideline they are following.
- The usual level of significance accepted is p ≤ 0.05. “Fisher suggested a probability of one in twenty (0.05) as a convenient cutoff level to reject the null hypothesis.” “The null hypothesis is the default assumption that nothing happened or changed”
(https://en.wikipedia.org/wiki/Statistical_significance). Note that “p values do not give the probability that a null hypothesis is false, they give the probability of obtaining data at least as extreme as those observed if the null hypothesis was true. It is by convention that smaller p values are interpreted as stronger evidence that the null hypothesis is false” (Woolston C. Psychology journal bans p values. Nature 2015;519:9).
- We can still apply tests and report p-values—it is a valid method, just don’t label results as “statistically significant”, but rather report how strong an effect is, and what this means or implies. As summarized in the ASA statement on p-values: “Good statistical practice, as an essential component of good scientific practice, emphasizes principles of good study design and conduct, a variety of numerical and graphical summaries of data, understanding of the phenomenon under study, interpretation of results in context, complete reporting, and proper logical and quantitative understanding of what data summaries mean. No single index should substitute for scientific reasoning” (Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat 2016:70:129–133).
- “Questionable research practices (QRPs) in the statistical analysis of data and in the presentation of the results in research papers include HARKing, cherry-picking, p-hacking, fishing, and data dredging or mining. HARKing (Hypothesizing After the Results are Known) is the presentation of a post hoc hypothesis as an a priori hypothesis. Cherry-picking is the presentation of favorable evidence with the concealment of unfavorable evidence. P-hacking is the relentless analysis of data with an intent to obtain a statistically significant result, usually to support the researcher’s hypothesis. A fishing expedition is the indiscriminate testing of associations between different combinations of variables not with specific hypotheses in mind but with the hope of finding something that is statistically significant in the data. Data dredging and data mining describe the extensive testing of relationships between a large number of variables for which data are available, usually in a database. This article explains what these QRPs are and why they are QRPs. This knowledge must become widespread so that researchers and readers understand what approaches to statistical analysis and reporting amount to scientific misconduct” (Andrade C. HARKing, cherrypicking, P-hacking, fishing expeditions, and data dredging and mining as questionable research practices. J Clin Psychiatry 2021;82:20f13804).
3.3 Adequacy of title, references, figures, and tables
The title must adequately reflect the contents of the manuscript. References must be as current and complete as possible but avoid the use of multiple references to back up a single fact. Figures and tables must be pertinent to the contents of the manuscript and must not be redundant with information already presented in the text.
4 Manuscript preparation
□ Please take the time to read our Best Practices for submitting, reviewing, and publishing in JVDI.
□ Microsoft Word file, double-spaced, 12-point Times New Roman font, left-justified, 25 mm (1 in.) margin on all sides, pages numbered at the bottom center (i.e., Page X of Y).
□ For styles, do not use Heading 1, etc. Use the Normal style setting.
|
□ |
Number the text lines consecutively throughout the manuscript; begin page 1 with line 1; do not restart numbering on each subsequent page. |
|
□ |
Indent paragraphs and do not include spaces between paragraphs. |
|
□ |
Allow 1 space (not 2) after a word or period. |
|
□ |
Punctuation: comma or period before superscripts; semicolon after superscripts; no spaces before or between superscripts. |
|
□ |
Use page breaks (= Ctrl-Enter) rather than a series of hard returns to separate sections of text. |
|
□ |
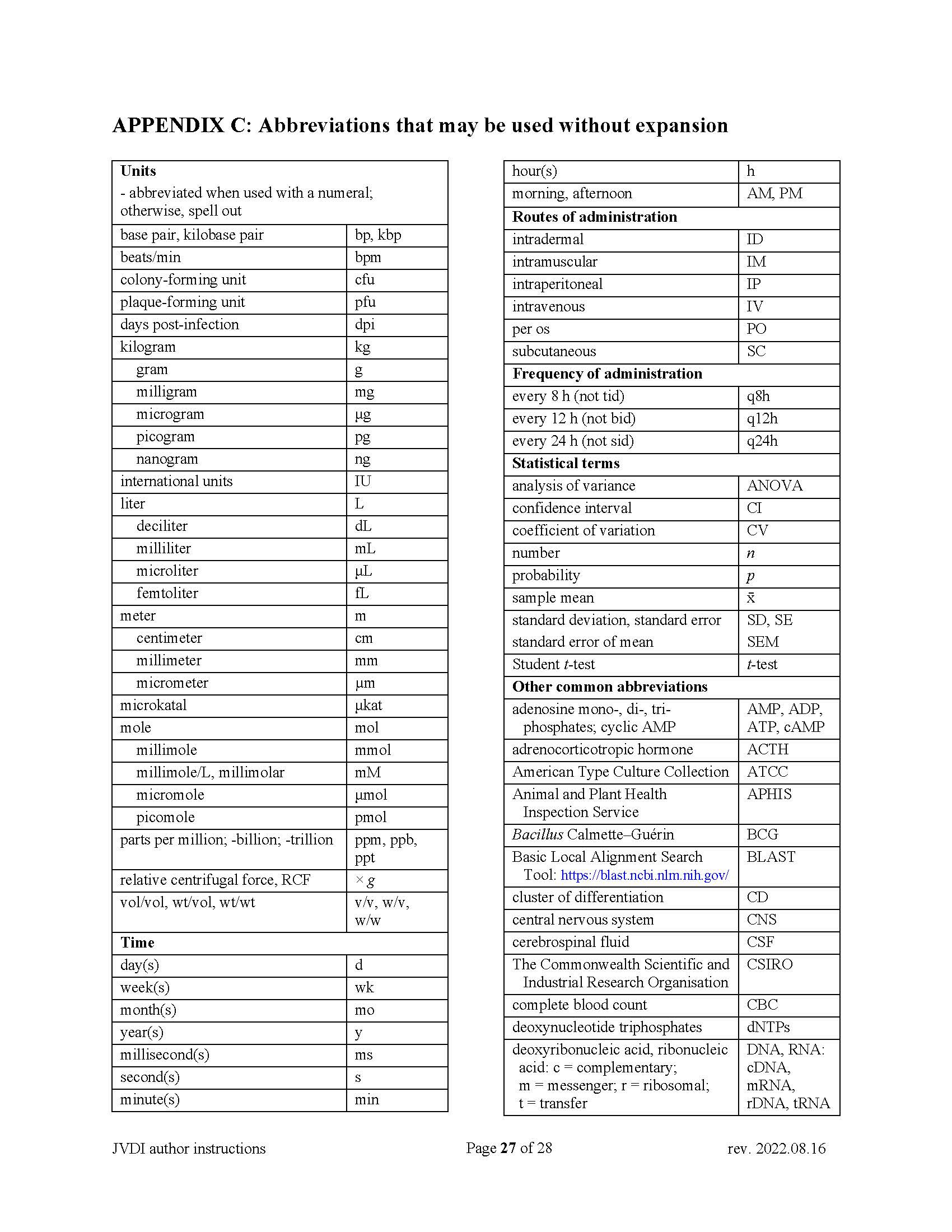
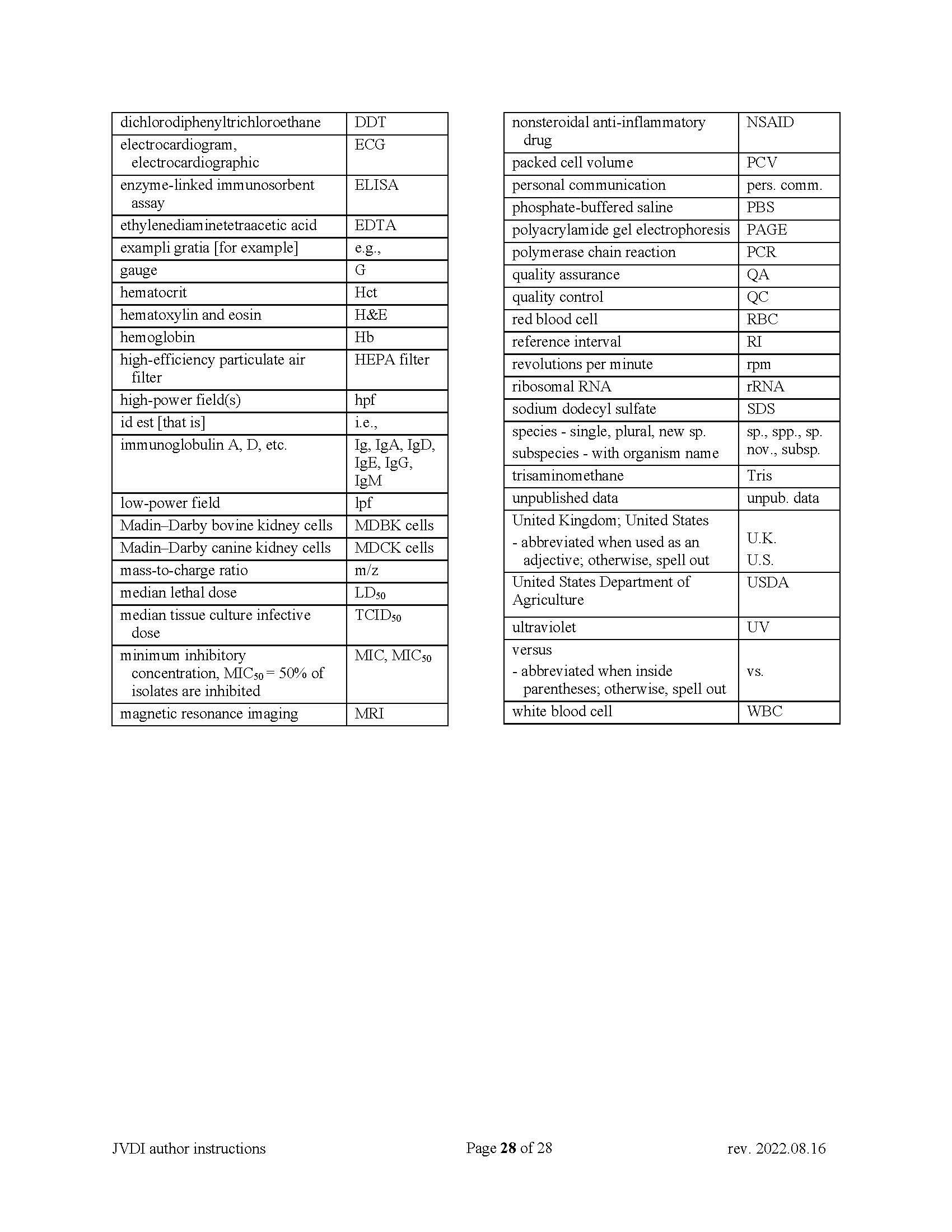
No need to capitalize words to abbreviate in capital letters; non-standard abbreviations must be defined on first use—note that the Abstract is independent of the main body of text. See Appendix C. |
|
□ |
Number style is one, 2, 3, 4… within the text, but 1, 2, 3, 4… when in a series in the same sentence. |
|
□ |
Number cases starting with case 1 regardless of your particular numbering system and use in all sections of the manuscript (e.g., cases 1–10; cases 2, 3, 5). Laboratory case numbers should not be used in the text, tables, or figures. |
|
□ |
SI units of measurement (International System of Units) must be used (may include conventional units in brackets; see section 4.1.3). |
|
□ |
For anatomic terms, use the English equivalents of terms in Nomina Anatomica Veterinaria. Names of infectious agents should follow the current published standards for viruses (ICTV, International Committee on Taxonomy of Viruses), bacteria (NCBI), and fungi (NCBI). |
|
□ |
Cite the brand and name of a manufacturer in parentheses at the appropriate location within the text. |
|
□ |
Reference citations in text are listed as superscripts after the punctuation, as shown.1,2-4,8 |
|
□ |
Arrange references alphabetically, numbered consecutively. |
|
□ |
Include tables in the main document, but do not embed figures. |
|
□ |
Submit figures as .tiff or .jpg only (see sect. 5). Do not exceed the max. file size of 5 MB per figure. |
|
□ |
Submit supplemental tables in a Microsoft Word file (see section 6). Submit supplemental figures following the figure guidelines (see section 5). |
4.1 General format and style
4.1.1 Layout and media
Three manuscript formats are accepted: Reviews, Full Scientific Reports, and Brief Reports (it is strongly encouraged that Brief Reports include a literature review). Supplemental files may be submitted with each of these formats (see sect. 6). Review articles are encouraged provided they cover subjects of current and broad interest to veterinary laboratory diagnosticians. Authors interested in submitting a Review should contact the Editor-in-Chief (gmaxie@uoguelph.ca).
The suitability of Letters to the Editor, Book Reviews, and Commentaries is determined by the Editorin-Chief, and a pre-submission inquiry to the editor is recommended. Book Reviews should be emailed to Donal O’Toole, the Book Review Editor (DOT@uwyo.edu), and not submitted through the submission site.
4.1.2 Language and style
The American form of English must be used, and manuscripts must be written in a style following the current standards for scientific publications. Editors will reject manuscripts that do not meet a minimum standard for written English. Abbreviations may be used after the first mention with complete spelling; standard abbreviations may be used without definition (see Appendix C). Use Arabic numerals except when a number begins a sentence, in which case spell the number out in full. JVDI number style is one, 2, 3, 4… within the text, but 1, 2, 3, 4… when in a series in the same sentence.
An editing service, including translation from Spanish, Portuguese, or Chinese, is available from Sage; use of this service does not guarantee acceptance of the manuscript by the journal.
“Use of the terms first world/third world and developed/developing are not recommended as descriptors when comparing countries or regions. Low-income, limited-income, resource-limited, resource-poor, transitional…” are acceptable (AMA Manual of Style, 11 ed.).
JVDI has accepted the practice of italicizing not only the names of family, genus, and species, but the formal scientific names of all taxa regardless of rank, as is the practice in the International Code of Nomenclature for algae, fungi, and plants (Thines M, et al. Setting scientific names at all taxonomic ranks in italics facilitates their quick recognition in scientific papers. IMA Fungus 2020;11:25).
4.1.3 Units of measurement
SI units of measurement (International System of Units) must be used (may include conventional units in brackets). Express centrifugal speed in relative centrifugal force (× g) and not in revolutions per minute. See Appendix C for abbreviations.
For easy conversions, see AMA Manual of Style: https://academic.oup.com/amamanualofstyle/si-conversion-calculator
For veterinary lab examples, see: https://www.uoguelph.ca/ahl/content/hematology-reference-intervals https://www.uoguelph.ca/ahl/biochemistry-reference-intervals
4.1.4 Tumor pathology
JVDI supports the need for standardization of tumor assessment (Meuten DJ, et al. International guidelines for veterinary tumor pathology: a call to action. Vet Pathol 2021;58:766–794; Schulman FY, et al. Reporting guidelines for manuscripts on tumor prognosis. Vet Pathol 2022. doi:10.1177/03009858221082207); checklists are available online in the Supplemental material that accompanies this article. Submitters are encouraged to follow these guidelines.
Report mitotic activity as mitotic count (MC) and not mitotic index (MI; Meuten DJ, et al. Mitotic count and the field of view area: time to standardize. Vet Pathol 2016;23:1–15). In a cellular region at the periphery of the tumor with the most mitotic activity, count the total number of mitotic figures in 2.37 mm2 (10 contiguous fields, no overlapping, high-power field [hpf] 40× objective, 10× ocular field number [FN] 22 mm, field of view [FOV] diameter 0.55 mm at specimen level), avoiding and/or skipping areas of the tumor that are cell-poor from hemorrhage, edema, necrosis, cysts, etc.
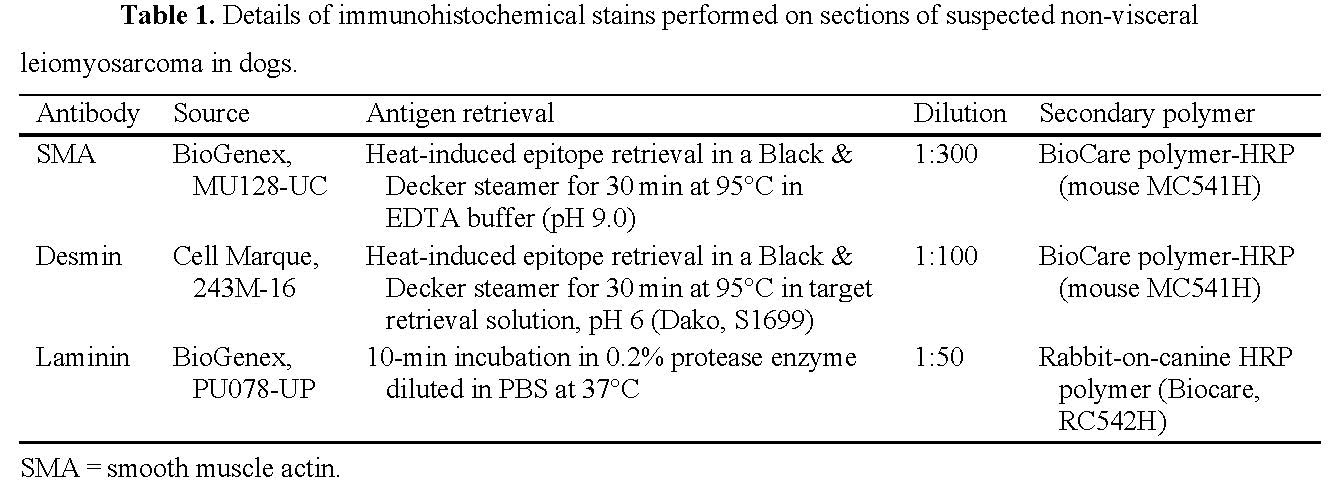
4.1.5 Immunohistochemistry
IHC tests reported must be validated in the subject species. Steps are best presented in table format for the various antibodies used, and include primary antibody (target antigen, host, source, clone, dilution), antigen retrieval method (heat-induced epitope retrieval, protease retrieval, no retrieval, other), chromogen, and detection system used (Caswell JL, et al. Observational study design in veterinary pathology, part 2: methodology. Vet Pathol 2018;55:774–785; Ramos-Vara JA, et al. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagn Invest 2008;20:393–413; Ramos-Vara JA, Miller MA. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry—the red, brown, and blue technique. Vet Pathol 2014;51:42–87).
Example 1. Robson HR, et al. Type A thymoma in a pet rabbit. J Vet Diagn Invest 2022;34:327–330.

Example 2. Brady RV, et al. Retrospective immunohistochemical investigation of suspected non-visceral leiomyosarcoma in dogs. J Vet Diagn Invest 2022:34:465–473.
4.1.5 Sources and manufacturers
Cite the name of the manufacturer in parentheses at the appropriate location within the text—addresses of suppliers are not required. Use generic names of drugs in the text, with the brand name in parentheses. No need to use ® or ™. No need for Corp., Inc., Ltd., Co., Pty., GmbH, AG, etc. No need for catalog numbers. For free online analyses or software, add the URL in parentheses.
Examples:
The Microflex LT MALDI-TOF mass spectrometer (Bruker Daltonics) was used in our study. The mass spectrometer was calibrated for molecular weights with a range of 3,637–16,952 Da prior to sample testing using the bacterial test standard (Bruker Daltonics), as per the manufacturer’s recommendations.
The amplicons were then purified, sequenced, and further confirmed by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
4.2 Detailed manuscript layout
4.2.1 Title page
Manuscript page 1: Title of paper, authors’ first name, middle initial, last name (e.g., John D. Doe); name and location of each author’s institution(s); name, postal address, and email of the corresponding author; and a short running head not to exceed 60 characters (including spaces; when published, the first author’s last name + et al. will appear on even-numbered pages and the short running head on odd-numbered pages). Please set up title and authors as in a recent issue of JVDI. Use sentence case for Article title.
Example:
Paeniclostridium (Clostridium) sordellii–associated enterocolitis in 7 horses
Akinyi C. Nyaoke, Mauricio A. Navarro, Karina Fresneda, Santiago S. Diab, Janet Moore, Dena Lyras, Milena Awad, Francisco A. Uzal1
California Animal Health and Food Safety Laboratory System, University of California–Davis, San Bernardino (Nyaoke, Navarro, Fresneda, Moore, Uzal) and Davis (Diab) branches, CA, USA; Infection and Immunity Program, Monash Biomedicine Discovery Institute and Department of Microbiology, Monash University, Clayton, Victoria, Australia (Lyras, Awad).
1Corresponding author: Francisco A. Uzal, California Animal Health and Food Safety, University of California–Davis, 105 W Central Ave, San Bernardino, CA 92408, USA. xxx@ucdavis.edu
Running head: Paeniclostridium sordellii enterocolitis in horses
4.2.2 Abstract
Manuscript page 2: Limit the abstract to 250 words or fewer (Full Scientific Reports) or 200 words or fewer (Brief Reports) and write as a single paragraph. It must be factual and concise, yet complete enough to stand alone without reference to the text. State conclusions clearly: “Results are discussed.” is unacceptable. Do not use reference citations in the Abstract.
4.2.3 Keywords
Manuscript page 2: Using terms from the medical subject headings (MeSH) list of the U.S. National Library of Medicine, provide an alphabetical list of keywords or phrases not to exceed 80 characters (including spaces). Note keywords directly below, and on the same page as, the Abstract. Spell out nonstandard abbreviations at first use. During online submission, Sage Track limits the number of keywords that can be submitted; this has no bearing on the list included in your manuscript.
4.2.4 Manuscript sections
- Reviews: Abstract (≤250 words) and Keywords; Introduction; and appropriate section headings and subheadings chosen by the author.
- Full Scientific Reports: Abstract (≤250 words) and Keywords; Introduction (no header needed); Materials and methods; Results; Discussion; Acknowledgments (optional); Declaration of conflicting interests; Funding; References; Tables (optional); and Figure legends (if applicable). Do not use subheads in Introduction and Discussion. Do not summarize your findings (In conclusion…) at the end of the manuscript (avoid a repeat of the Abstract).
- Brief Reports: Abstract (≤200 words) and Keywords; body of manuscript (<2,000 words; no section or subheadings in the main text); Acknowledgments (optional); Declaration of conflicting interests; Funding; References (n ≤ 20); Tables (optional); and Figure legends (if applicable; note: usually no more than 1 plate of 4 figures). Do not summarize your findings (In conclusion…) at the end of the manuscript (avoid a repeat of the Abstract).
- Supplemental material is optional but welcomed (see section 6).
Acknowledgments
In the Acknowledgments section, list all contributors who do not meet the criteria for authorship. Examples of those who might be acknowledged include a person who provided purely technical help, writing assistance, or a department chair who provided only general support. Authors are to disclose whether they had any writing assistance and identify the entity that paid for this assistance.
Artificial Intelligence
Use of Large Language Models and generative AI tools in writing your submission
Sage recognizes the value of large language models (LLMs) (e.g. ChatGPT) and generative AI as productivity tools that can help authors in preparing their article for submission; to generate initial ideas for a structure, for example, or when summarizing, paraphrasing, language polishing etc. However, it is important to note that all language models have limitations and are unable to replicate human creative and critical thinking. Human intervention with these tools is essential to ensure that content presented is accurate and appropriate to the reader. Sage therefore requires authors to be aware of the limitations of language models and to consider these in any use of LLMs in their submissions:
- Objectivity: Previously published content that contains racist, sexist or other biases can be present in LLM-generated text, and minority viewpoints may not be represented. Use of LLMs has the potential to perpetuate these biases because the information is decontextualized and harder to detect.
- Accuracy: LLMs can ‘hallucinate’ i.e. generate false content, especially when used outside of their domain or when dealing with complex or ambiguous topics. They can generate content that is linguistically but not scientifically plausible, they can get facts wrong, and they have been shown to generate citations that don’t exist. Some LLMs are only trained on content published before a particular date and therefore present an incomplete picture.
- Contextual understanding: LLMs cannot apply human understanding to the context of a piece of text, especially when dealing with idiomatic expressions, sarcasm, humor, or metaphorical language. This can lead to errors or misinterpretations in the generated content.
- Training data: LLMs require a large amount of high-quality training data to achieve optimal performance. However, in some domains or languages, such data may not be readily available, limiting the usefulness of the model.
Guidance for authors
Authors are required to:
- Clearly indicate the use of language models in the manuscript, including which model was used and for what purpose. Please use the methods or acknowledgements section, as appropriate.
- Verify the accuracy, validity, and appropriateness of the content and any citations generated by language models and correct any errors or inconsistencies.
- Provide a list of sources used to generate content and citations, including those generated by language models. Double-check citations to ensure they are accurate, and are properly referenced.
- Be conscious of the potential for plagiarism where the LLM may have reproduced substantial text from other sources. Check the original sources to be sure you are not plagiarizing someone else’s work.
- Acknowledge the limitations of language models in the manuscript, including the potential for bias, errors, and gaps in knowledge.
- Please note that AI bots such as ChatGPT should not be listed as an author on your submission.
We will take appropriate corrective action where we identify published articles with undisclosed use of such tools.
Conflicting interests
Required section. A statement is required from all authors to be carried within the paginated pages of all published articles, under the heading “Declaration of conflicting interests”. If no conflicting interests exist, please use the following text:
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
When making a declaration, the disclosure information must be specific and include any financial relationship that authors of the article have with any sponsoring organization and the for-profit interests the organization represents, and with any for-profit product discussed or implied in the text of the article.
Any commercial or financial involvements that might represent an appearance of a conflict of interest need to be additionally disclosed in the cover letter accompanying your article to assist the Editor in evaluating whether sufficient disclosure has been made within the Declaration of Conflicting Interests provided in the article. For more information, please visit the Sage Journal Author Gateway.
Publication of papers dealing with a commercial product or laboratory test does not convey or imply an endorsement by the Journal of Veterinary Diagnostic Investigation or the American Association of Veterinary Laboratory Diagnosticians.
Funding
Required section. Disclose any funding sources, as well as grant numbers. If no outside funding was used, please use the following text:
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
In the text, identify references with superscript numbers. Do not use names of authors in the text. Authors are responsible for the accuracy of all references.
Examples:
Studies1,3,25 have shown….
Rarely, the nasal infection caused by P. insidiosum in sheep extends to the rhinopharyngeal region31; the submandibular and parotid lymph nodes are almost always involved.25,27,31
In the list of References, first arrange references alphabetically by the first author, then number them consecutively (see Appendix B).
Personal communications should be listed in the text [e.g., (Maxie MG, pers. comm., 2020 Apr 08)].
Tables
Tables should appear on separate pages following the References. The table title appears directly above the table. Table titles must be free-standing and self-explanatory. Number all tables consecutively with Arabic numerals and cite consecutively in the text. Insert citations in the text following relevant results (Table 1, Fig. 3), rather than devoting a sentence to referencing the content of the Table or Figure (“… are presented in Table 1.”).
Layout: left-align columns; top justify cells; sentence case for column heads and entries; set title and table footnotes as text outside of the table; no bolding other than to show abnormal values. Spell out all abbreviations in alphabetical order using table footnotes (e.g., M = male; ND = not determined). Indicate references to items other than abbreviations using the following sequential symbols (*, †, ‡, §, ¦, #, ¶, **).
See example below, as well as the examples in section 4.1.5.
Example:
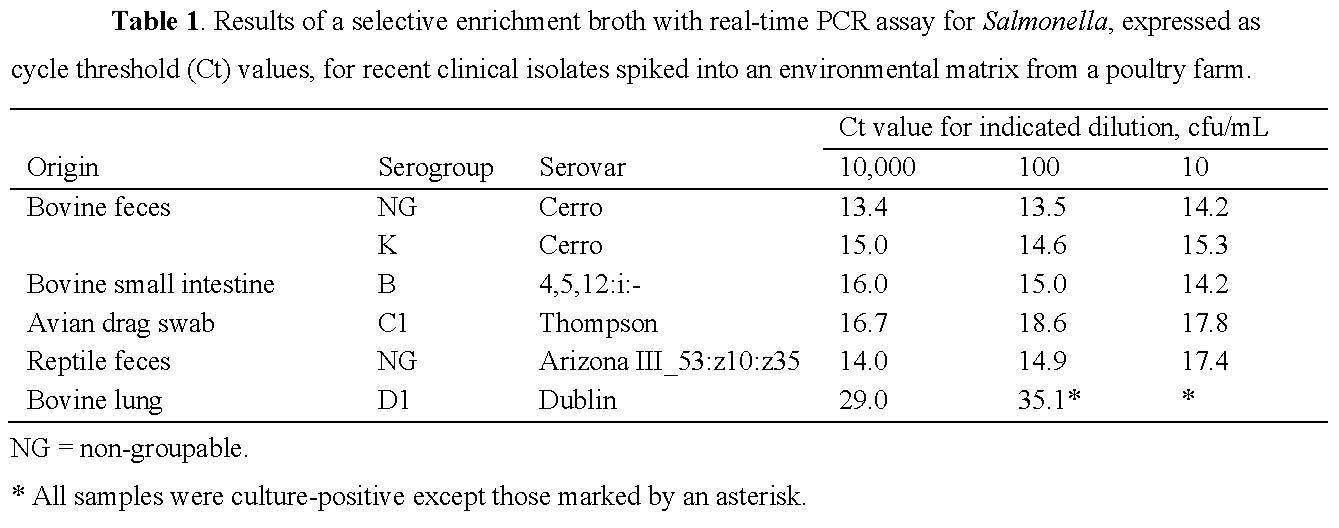
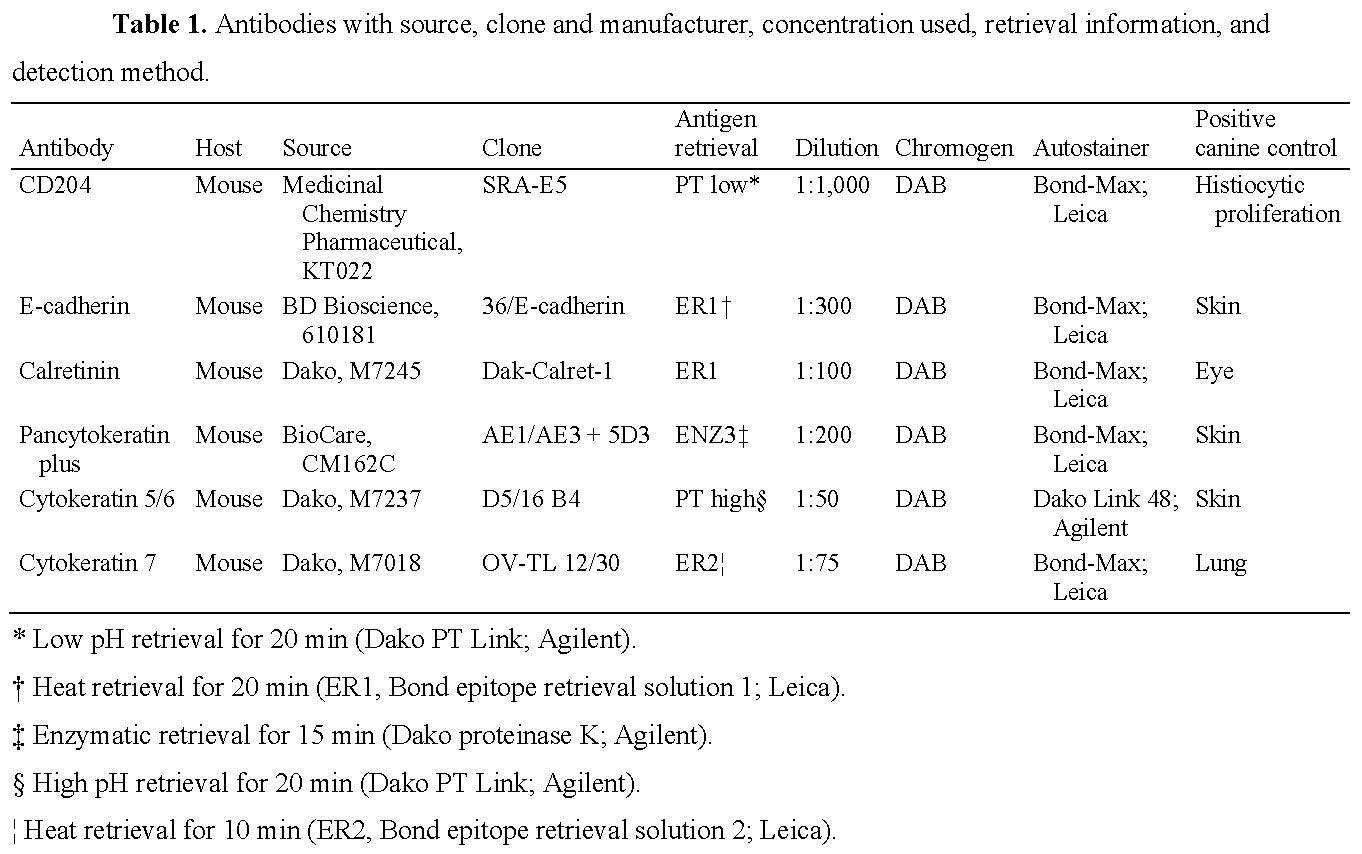
Figure legends
Every figure must have a corresponding legend. Legends appear on a separate page following the References or Tables. Identify animal species, organ, or tissue, and describe the figure in a continuous phrase or sentence. Indicate stains on light and electron micrographs within the figure legend. See also Image magnification in section 5. Related series of figures may be grouped into plates (see Panel image alignment in section 5).
Example of single-panel image legend:
Figure 1. Granulomatous hepatitis in a llama. H&E. Bar = 20 μm.
Example of multiple-panel image (plate) legend:
Figures 1–4. Membranous ventricular septal aneurysm in a black-tailed prairie dog. Figure 1. A multinodular dark red mass with a smooth and shiny surface projects into the lumen of the right ventricle (RV) from the interventricular septum in the area of the septal cusp of the tricuspid valve. Black line indicates plane of section in Figs. 2–4. Figure 2. Longitudinal section of the heart through the mass including right atrium (RA), right ventricle, and left ventricle (LV), showing an absent membranous interventricular septum (arrow) with a thin wall separating the left and right ventricles. This wall is formed by the septal cusp of the tricuspid valve, the free edge of which is adherent to the interventricular septum. A large lamellated recent thrombus fills the aneurysmal space (asterisk). H&E. Figure 3. The septal cusp of the tricuspid valve separating the left and right ventricular lumina is strongly alcianophilic on the atrial aspect, similar to the aortic (top left) and parietal tricuspid (top center) valve cusps. Inset: higher magnification of the cusp. Alcian blue and periodic acid–Schiff. Figure 4. The septal cusp of the tricuspid valve separating the left and right ventricular lumina is densely collagenous on the ventricular aspect, with a loose collagenous matrix on the atrial aspect, similar to the aortic (top left) and parietal tricuspid (top center) valve cusps. Inset: higher magnification of the cusp. Masson trichrome.
5 Figures
Failure to submit figures in the required format and resolution will result in immediate rejection. See our Best Practices (slides 19–29) for tips on creating and modifying figures. Figures must NOT be embedded in the text document but submitted separately. Please submit figures UN-flattened to allow for editing if needed. Do not exceed the maximum file size of 5 MB per figure.
Acceptable formats are .tiff and .jpg
- minimum acceptable resolution is 300 dpi (300 pixels/inch, 118.11 pixels/cm) for photographs, pathology images
- minimum acceptable resolution is 600 dpi (600 pixels/inch, 236.22 pixels/cm) for line art (e.g., graphs, charts, maps)
Sizing
- Single illustrations should be one column wide (90 mm wide)
- Illustrations with figure panels should be reproduce in 2 columns (180 mm wide) or 1 column (90 mm wide). See panel image alignment below.
Group multiple color figures into a composite figure separated by a thin white line, with the individual panels clearly identified (i.e., 1, 2, 3, etc. for different lesions or organs; or A, B, C, etc. for closely related images, e.g., various IHCs of similar sections) in 14-point Arial, located in the bottom left corner of each image. Save figures as separate files with the figure number (Fig 1, Fig 2, Figs 3–5, etc.) as the file name (do not use figure numbers and/or titles as part of the image).
Submit pathology images in color.
- In gross pathology images, grass, surgery drapes, bodily fluids, gloves, etc., must be digitally removed and replaced with a more-or-less uniform background.
- In gross anatomic photographs, locate the head to the right of the image. o Scale bars, case numbers, or other identification legends are not needed.
- In photomicrographs, orient the surface of the skin or mucous membrane at the top of the figure. Photomicrographs must not contain photographic or tissue artifacts, and the images must be evenly lit with white backgrounds (places where there is no tissue). Limit image modification or enhancement to that obtainable by ordinary photographic techniques or to adjustment of white balance, brightness, or contrast by photo-editing software. If these parameters in your figures are unacceptable, the Images editor may make suitable adjustments.
- There is no requirement to indicate image size in the figure legend.
- Scale bars may be used if deemed necessary by the authors but are not required. Scale bars may be black or white (depending on the background) and are located on the bottom right corner of the image. Please do not place the μm size on the figure or the bar; instead, add the size to the figure legend (e.g., Bar = 20 μm).
- Electron micrographs must have a scale bar in the figure.
Panel image alignment
Set your panel in the form of a rectangle or square, with no empty spaces, except for the thin white lines between images. Panels may have up to 3 figures across and up to 3 figures vertical.
3 examples of acceptable panel layouts.

If you submit your images as .tiff files, use LZW compression. LZW compression will reduce the file size (sometimes dramatically) without affecting quality. When saving a file such as a .tiff from any photoediting program, you are given the option of compressing the file. LZW compression will speed up the upload and download times and will not affect your image negatively.
Line art guidelines
See our Best Practices (slides 30–31) for tips on creating and modifying line art. All text in figures
(Arial, in Sentence case) must be of sufficient point size to be readable in print.
6 Supplemental files
If your manuscript contains supplemental data, please add the following text in the main document following the Funding section. All supplemental data must be referenced in the text.
Supplemental material
Supplemental material for this article is available online.
Example:
Laboratories were asked to provide a list of 10 most common bacterial isolates on which they performed antimicrobial susceptibility testing across all animal species (Table 2; Suppl. Table 1).
The JVDI editorial office edits any submitted supplemental material (submit text and tables as .doc files; submit figures as .tiff or .jpg files) for formatting and consistency with the main document, then combines the material into a single PDF file for posting. Do not submit supplemental material as pdfs.
JVDI, through Sage Publications, hosts supplemental materials online, alongside the full text of articles. Supplemental materials are generally files that were used to create the research (e.g., datasets, dendrograms, etc.) or additional pieces to the article that are not included in the fee version (such as audio/video material, tables, figures, or raw data).
7 New manuscript submission
- Submit all parts of the manuscript via Sage Track.
- If you have previously submitted or reviewed a manuscript using Sage Track, use your existing User ID and Password to log in. NOTE: If you are not registered, click “Create an Account” and follow the on-screen instructions.
—Your ORCID iD will not be published in your article unless you link your existing iD with your ScholarOne account in Sage Track. We cannot add/amend ORCID iDs for any article at the proof stage. To edit your ScholarOne account, login to Sage Track and click E-mail/Name from the dropdown menu under your name, which appears in the upper right corner of your screen.
—If you do not have an ORCID iD, you are provided with a link to create an account.

- Click on the Author Center, and follow the submission process, providing information when prompted.
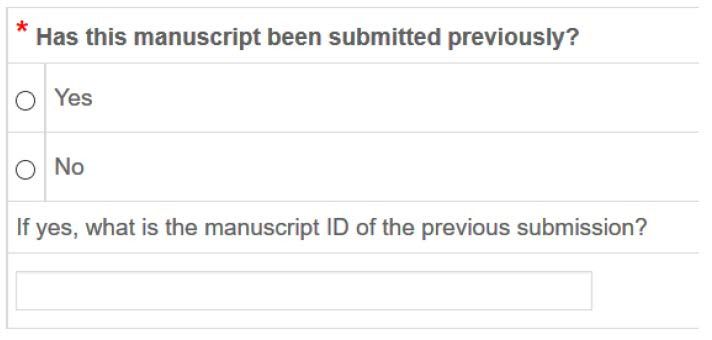
- As shown in the screenshot below, if you are resubmitting a previously rejected manuscript, you must answer YES and enter your previous manuscript number during the submission process. If you do not know your previously assigned manuscript number, please contact the Editorial Office.
- Please ensure that all authors have been entered in the submission process to ensure that Journal correspondence is sent to all authors. If your coauthors wish for their ORCID iD to be published in your article, they must login to their ScholarOne account at Sage Track to create an ORCID iD or associate their existing ORCID iD.
NOTE: For new and revised manuscript submissions, our submission site generates a pdf of your files (main document, figures, suppl. material) at the end of your submission. You are required to check the pdf to verify that all of your files have uploaded successfully and that the files are in the correct order of appearance. The pdf will display your figures at a smaller size and lower resolution to help the pdf load faster. Your original files are not altered during this process. The Images editor evaluates your figures from your original files, and not from the pdf.
8 Revised manuscript submission
- Use your User ID and Password to log in to Sage Track.
- Click on Author Center, then Manuscripts with Decisions.
- Locate the manuscript you wish to revise and click Create a Revision.
- You will be prompted to respond to the comments made by the Editor and/or Reviewers. Important: Address each comment and detail your changes in the space provided or by uploading a Word document with the reviewers’ comments listed and your response to each.
- Follow the submission process, providing information when prompted.
All submissions are reviewed by the Editor-in-chief and may be rejected based on content and/or formatting. Suitable manuscripts are assigned by the EIC to a Section editor, who will oversee the peerreview process. Once peer-reviewed, the revised version of the manuscript is forwarded to the EIC for review and approval (further revision is often required). In the meantime, photo images are reviewed by the Images editor. The EIC then sends the approved manuscript to the Managing editor for copyediting where it will be held in the category “Awaiting Editor Decision” in Sage Track until edited. After final approval of the copyediting by the EIC and author, the manuscript files are forwarded to Sage Publications.
9 Page proofs
The Corresponding Author will receive page proofs by email from the Sage Production Editor within 2– 4 wk from time of acceptance by the Managing editor.
10 Help
If you experience any problems during the online submission process, please consult the Sage Track Author Guide and FAQs, which provides detailed submission instructions. Holly Farrell, the Managing editor (editorial@aavld.org), is also available for assistance. Francisco Uzal, the Images editor (images@aavld.org), is available for specific questions about figures.
APPENDIX A: PCR guidelines—suggested dossier or publication information
APPENDIX A: PCR guidelines—suggested dossier or publication information
Sample
Source of known positive and negative samples
Description of sample types and species
Processing procedure
Description of procedure and length of time, if frozen
Name of fixative and length of time, if fixed
Sample storage conditions and duration (especially for FFPE samples)
Nucleic acid extraction
Procedure and/or instrumentation
Name of kit and details of any modifications
Source of additional reagents used
Specify internal control
PCR target information
Name of target gene
Sequence accession number
Coordinates according to accession number and length of amplicon
In silico specificity screen (BLAST), secondary structure analysis, sequence alignment; all are not
necessary to show but author should indicate it has been done
Primer sequences, unless evaluating a commercial kit with proprietary information
Primer/probe source
PCR amplification
Primer/probe concentration given in µm in final reaction
Master mix reagents (buffer, enzyme, Mg, dNTPs)*
Additives*
Reaction volume
Complete thermocycling parameters
Manufacturer of qPCR instrument
Controls included: NTC, internal control, amplification control
Analysis methods and software version
Analytical performance characteristics
Limit of detection (LOD)
Efficiency
Linearity
Range
Repeatability
Diagnostic performance characteristics
Table adapted from MIQE (Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–622).
* Include vendor/manufacturer.
APPENDIX B: Reference formats
APPENDIX B: Reference formats
Journal articles
More than 2 authors
Solanki AK, et al. Clostridium perfringens beta toxin DNA prime-protein boost elicits enhanced protective immune response in mice. Appl Microbiol Biotechnol 2017;101:5699–5708.
In language other than English
Trindade AB, et al. Lipossarcoma em caturrita (Myiopsitta monachus) [Liposarcoma in a caturrita (Myiopsitta monachus)]. Cienc Anim Bras 2010;11:971–976. Portuguese.
In a supplement
Schultz RD, et al. Age and long-term protective immunity in dogs and cats. J Comp Pathol 2010;142(Suppl 1):S102–S108.
Forthcoming
Clift SJ, et al. Polyclonal antibody–based immunohistochemical detection of intra-leukocytic Theileria parasites in roan and sable antelopes. J Vet Diagn Invest 2021. Accepted.
Epub ahead of print
Webster JD, et al. Validating immunohistochemistry assay specificity in investigative studies: considerations for a weight of evidence approach. Vet Pathol. Published online 2020 Sept 25. doi:10.1177/0300985820960132
Errata/Corrigendum
Ong CB, Herdt TH, Fitzgerald SD. Hyperplastic goiter in two adult dairy cows. J Vet Diagn Invest 2014;26:810–814. Corrigendum: J Vet Diagn Invest 2018;30:654.
Books
Chapter in a book
Craig LE, et al. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. Elsevier, 2016:16–163.
Book in a series
Koestner A, et al. Histological Classification of Tumors of the Nervous System of Domestic Animals. 2nd series. (Vol. 5, WHO International Classification of Tumors of Domestic Animals). Armed Forces Institute of Pathology, 1999.
Other media
CLSI document
Clinical and Laboratory Standards Institute (CLSI). Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. CLSI, 2010. CLSI document EP28-A3C3.
Conference proceedings (add page numbers and URL if available)
Reed KF, et al. Milk urea nitrogen: precision, accuracy, and individual animal variability. In: Proc 82nd Cornell Nutr Conf; Ithaca, NY; 2020:192–200. https://hdl.handle.net/1813/72919
Stoltsz WH, Dunsterville MT. In vitro establishment and cultivation of a Cytauxzoon sp. (Theileria sp.) from a sable antelope (Hippotragus niger, Harris 1838). J S Afr Vet Assoc 1992;63:182. Parasitol Soc South Africa abstract. https://journals.co.za/doi/pdf/10.10520/AJA00382809_2977
Dissertation/thesis (add URL if available)
Roguskie JM. The role of Pseudomonas aeruginosa 1244 pilin glycan in virulence. Master thesis. Duquesne University, 2005. https://dsc.duq.edu/etd/1119
Zehr ESN. Relatedness of Haemophilus parasuis strains and their proteins’ possible roles as virulence factors. PhD dissertation. Iowa State University, 2008.
European Commission
European Commission. Commission Decision (2002/657/EC) of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun 2002;L221:8–36.
Newspaper article
Gaul G. When geography influences treatment options. Washington Post (Maryland Ed.). 2005 Jul 24:Sect. A:12 (col. 1).
Online database
Elliott-Smith E, Haig SM. Piping plover. 2020 March 4. Version 1.0. Birds of the World. Cornell Laboratory of Ornithology. https://birdsoftheworld.org/bow/species/pipplo/cur/introduction
Patent
Pagedas AC, inventor; ABS Inc., assignee. Flexible endoscopic grasping and cutting device and positioning tool assembly. United States patent US 20020103498. 2002 Aug 1.
Preprint
Hamer SA, et al. Natural SARS-CoV-2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID-19 cases in Texas, USA. bioRxiv 2020:2020.12.08.416339. doi:10.1101/2020.12.08.416339. Preprint.
Scientific report
Barker B, Degenhardt L. Accidental drug-induced deaths in Australia 1997–2001. University of New South Wales, National Drug and Alcohol Research Centre, 2003.
Kock TJ, et al. Evaluation of water temperature effects on adult sockeye salmon (Oncorhynchus nerka) behavior in the Yakima River, Washington, 2019. U.S. Geological Survey, 2020. Open-file report 2020-1033.
Simpson VR. Health status of otters in southern and south west England 1996–2003. Environment Agency, 2007. Science report SC010064/SR1.
URL
American Veterinary Medical Association. SNOMED, HL7, LOINC the official informatics standards for veterinary medicine. 2002 May 15. https://www.avma.org/javma-news/2002-06-01/snomed-hl7-loinc-official-inf...
Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Cotham and Salmonella Kisarawe infections linked to contact with pet bearded dragons (final update). 2014 Aug 20. https://www.cdc.gov/salmonella/cotham-04-14/index.html
Institute of Medicine of the National Academies. Key capabilities of an electronic health record system. Letter report. The National Academies Press, 2003. https://www.nap.edu/catalog/10781/key-capabilities-of-an-electronic-heal...
If publication date is not available, add access date
U.S. Department of Agriculture. U.S. cattle production. Accessed 2020 April 9: https://www.ers.usda.gov/topics/animal-products/cattle-beef/sector-at-a-...
APPENDIX C: Abbreviations that may be used without expansion